Example Of Bimolecular Reaction . a bimolecular reaction refers to a chemical reaction that typically occurs on a time scale slower than nanoseconds, involving the. the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. an elementary bimolecular reaction originates from a collision between two reactants. some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. bimolecular reaction involves the collision of two reactants a and. A bimolecular reaction involves the collision of two particles. The collision produces an activated. Bimolecular reactions are common in organic reactions such as. Very useful correlations between the. To yield two products c and d. Whether or not a collision. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide:
from chemistryjee.blogspot.com
an elementary bimolecular reaction originates from a collision between two reactants. some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. A bimolecular reaction involves the collision of two particles. some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: bimolecular reaction involves the collision of two reactants a and. The collision produces an activated. Very useful correlations between the. Whether or not a collision.
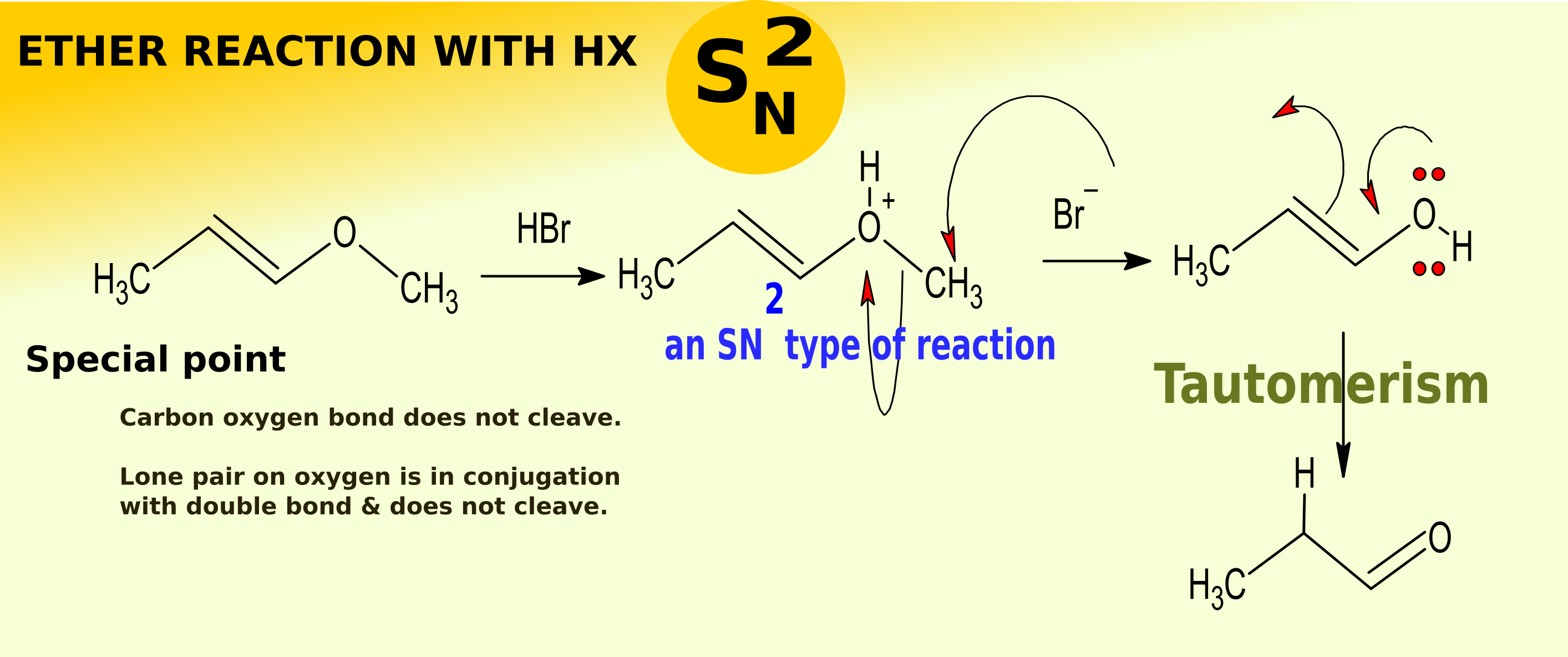
chemistry world Ether Cleavage reaction via substitution nucleophillic
Example Of Bimolecular Reaction A bimolecular reaction involves the collision of two particles. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: The collision produces an activated. a bimolecular reaction refers to a chemical reaction that typically occurs on a time scale slower than nanoseconds, involving the. some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. Very useful correlations between the. an elementary bimolecular reaction originates from a collision between two reactants. bimolecular reaction involves the collision of two reactants a and. the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. To yield two products c and d. Bimolecular reactions are common in organic reactions such as. Whether or not a collision. some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. A bimolecular reaction involves the collision of two particles.
From www.slideshare.net
Lect w4 152 rate and mechanisms_alg (1) Example Of Bimolecular Reaction some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. bimolecular reaction involves the collision of two reactants a and. The collision produces an activated. a typical example of a bimolecular process is the reaction between nitrogen dioxide and. Example Of Bimolecular Reaction.
From www.youtube.com
E2 Bimolecular Elimination Reactions YouTube Example Of Bimolecular Reaction some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. Very useful correlations between the. Bimolecular reactions are common in organic reactions such as. Whether or not a collision. the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. The collision produces an activated. some. Example Of Bimolecular Reaction.
From www.masterorganicchemistry.com
Chemical Master Organic Chemistry Example Of Bimolecular Reaction some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. Bimolecular reactions are common in organic reactions such as. The collision produces an activated. To yield two products c and d. an elementary bimolecular reaction originates from a collision between. Example Of Bimolecular Reaction.
From www.jamberoo.org
Studying Chemical Reactions with Gaussian Example Of Bimolecular Reaction Bimolecular reactions are common in organic reactions such as. the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. To yield two products c and d. Whether or not a collision. bimolecular reaction involves the collision of two reactants a and. Very useful correlations between the. an elementary. Example Of Bimolecular Reaction.
From www.slideserve.com
PPT Chemical “Rates of Reactions” PowerPoint Presentation Example Of Bimolecular Reaction Very useful correlations between the. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. bimolecular reaction involves the collision of two reactants a and. The collision produces an activated. Whether or. Example Of Bimolecular Reaction.
From www.youtube.com
How to Determine Molecularity of a Reaction Examples (Unimolecular Example Of Bimolecular Reaction a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: Bimolecular reactions are common in organic reactions such as. To yield two products c and d. The collision produces an activated. Very useful correlations between the. a bimolecular reaction refers to a chemical reaction that typically occurs on a time scale slower. Example Of Bimolecular Reaction.
From www.youtube.com
Bimolecular Reaction Mechanisms YouTube Example Of Bimolecular Reaction some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. Bimolecular reactions are common in organic reactions such as. an elementary bimolecular reaction originates from a collision between two reactants. The collision produces an activated. To yield two products c and d. A bimolecular reaction involves the collision of two particles. the reaction. Example Of Bimolecular Reaction.
From www.chemistrysteps.com
SN2 Reaction Mechanism Example Of Bimolecular Reaction some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. Whether or not a collision. Bimolecular reactions are common in organic reactions such as. bimolecular reaction involves the collision of two reactants a and. To yield two products c and d. an elementary bimolecular reaction originates from a collision between two reactants. a. Example Of Bimolecular Reaction.
From www.numerade.com
SOLVED Which of the following reactions involve carbocation as an Example Of Bimolecular Reaction a bimolecular reaction refers to a chemical reaction that typically occurs on a time scale slower than nanoseconds, involving the. Bimolecular reactions are common in organic reactions such as. To yield two products c and d. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: the reaction of oh with. Example Of Bimolecular Reaction.
From www.youtube.com
Nucleophilic Substitution Bimolecular Reaction YouTube Example Of Bimolecular Reaction the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. Very useful correlations between the. some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. a bimolecular reaction refers to a chemical reaction that typically occurs on a time scale slower than nanoseconds, involving the.. Example Of Bimolecular Reaction.
From byjus.com
what is the formula of bimolecular collision (z_11 Example Of Bimolecular Reaction an elementary bimolecular reaction originates from a collision between two reactants. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: To yield two products c and d. some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. the reaction of oh with ch4 forming h2o +. Example Of Bimolecular Reaction.
From www.researchgate.net
Parallelized unimolecular and bimolecular reactions. a Time profiles of Example Of Bimolecular Reaction Bimolecular reactions are common in organic reactions such as. some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. bimolecular reaction involves the collision of two reactants a and. To yield two products c and d. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: . Example Of Bimolecular Reaction.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free Example Of Bimolecular Reaction some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. an elementary bimolecular reaction originates from a collision between two reactants. Very useful correlations between the. bimolecular reaction involves the collision of two reactants a and. some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. Whether or not. Example Of Bimolecular Reaction.
From slideplayer.com
Chemical Derived Rate Laws from Reaction Mechanisms ppt download Example Of Bimolecular Reaction some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. a bimolecular reaction refers to a chemical reaction that typically occurs on a time scale slower than nanoseconds, involving the. the reaction of oh with ch4 forming h2o + ch3 is an example of a reaction of this class. an elementary bimolecular. Example Of Bimolecular Reaction.
From www.slideserve.com
PPT NUCLEOPHILIC SUBSTITUTION REACTIONS Part 1 PowerPoint Example Of Bimolecular Reaction Bimolecular reactions are common in organic reactions such as. Very useful correlations between the. bimolecular reaction involves the collision of two reactants a and. Whether or not a collision. a bimolecular reaction refers to a chemical reaction that typically occurs on a time scale slower than nanoseconds, involving the. To yield two products c and d. The collision. Example Of Bimolecular Reaction.
From www.researchgate.net
Graphical representation of the reversible bimolecular reaction model Example Of Bimolecular Reaction an elementary bimolecular reaction originates from a collision between two reactants. To yield two products c and d. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: Bimolecular reactions are common in organic reactions such as. bimolecular reaction involves the collision of two reactants a and. A bimolecular reaction involves. Example Of Bimolecular Reaction.
From www.chemistrylearner.com
Physical Chemistry Page 2 of 3 Chemistry Learner Example Of Bimolecular Reaction a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: an elementary bimolecular reaction originates from a collision between two reactants. some chemical reactions have mechanisms that consist of a single bimolecular elementary reaction. some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. A bimolecular. Example Of Bimolecular Reaction.
From www.numerade.com
SOLVED Order and rate law of a reaction The overall order of an Example Of Bimolecular Reaction some chemical reactions occur by mechanisms that consist of a single bimolecular elementary reaction. bimolecular reaction involves the collision of two reactants a and. a typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide: To yield two products c and d. The collision produces an activated. A bimolecular reaction involves the. Example Of Bimolecular Reaction.